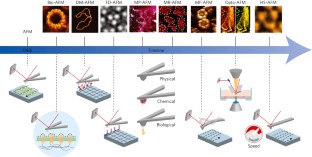
Imaging modes of atomic force microscopy for application in molecular and cell biology
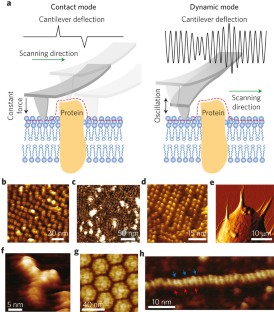
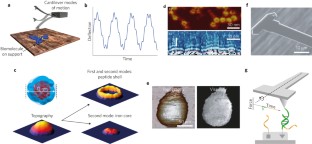
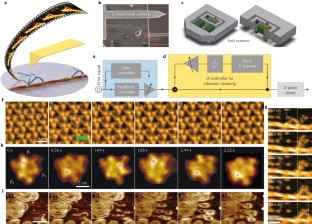
Atomic force microscopy (AFM) is a powerful, multifunctional imaging platform that allows biological samples, from single molecules to living cells, to be visualized and manipulated. Soon after the instrument was invented, it was recognized that in order to maximize the opportunities of AFM imaging in biology, various technological developments would be required to address certain limitations of the method. This has led to the creation of a range of new imaging modes, which continue to push the capabilities of the technique today. Here, we review the basic principles, advantages and limitations of the most common AFM bioimaging modes, including the popular contact and dynamic modes, as well as recently developed modes such as multiparametric, molecular recognition, multifrequency and high-speed imaging. For each of these modes, we discuss recent experiments that highlight their unique capabilities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
265,23 € per year
only 22,10 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Force spectroscopy of single cells using atomic force microscopy
Article 23 September 2021

Localization atomic force microscopy
Article 16 June 2021

Two-dimensional TIRF-SIM–traction force microscopy (2D TIRF-SIM-TFM)
Article Open access 12 April 2021
References
- Binnig, G., Quate, C. F. & Gerber, C. Atomic force microscope. Phys. Rev. Lett.56, 930–933 (1986). This paper first described the principles of AFM.ArticleCASGoogle Scholar
- Gerber, C. & Lang, H. P. How the doors to the nanoworld were opened. Nat. Nanotech.1, 3–5 (2006). CASGoogle Scholar
- Binnig, G., Gerber, C., Stoll, E., Albrecht, T. R. & Quate, C. F. Atomic resolution with atomic force microscope. Europhys. Lett.3, 1281–1286 (1987). CASGoogle Scholar
- Drake, B. et al. Imaging crystals, polymers, and processes in water with the atomic force microscope. Science243, 1586–1589 (1989). CASGoogle Scholar
- Radmacher, M., Tillmann, R. W. & Gaub, H. E. Imaging viscoelasticity by force modulation with the atomic force microscope. Biophys. J.64, 735–742 (1993). CASGoogle Scholar
- Horber, J. K. & Miles, M. J. Scanning probe evolution in biology. Science302, 1002–1005 (2003). CASGoogle Scholar
- Binnig, G. & Rohrer, H. In touch with atoms. Rev. Mod. Phys.71, S324 (1999). CASGoogle Scholar
- Muller, D. J. & Dufrene, Y. F. Atomic force microscopy as a multifunctional molecular toolbox in nanobiotechnology. Nat. Nanotech.3, 261–269 (2008). Google Scholar
- Muller, D. J. & Dufrene, Y. F. Atomic force microscopy: a nanoscopic window on the cell surface. Trends Cell Biol.21, 461–469 (2011). Google Scholar
- Hansma, H. G. & Hoh, J. H. Biomolecular imaging with the atomic force microscope. Annu. Rev. Biophys. Biomol. Struct.23, 115–139 (1994). CASGoogle Scholar
- Hinterdorfer, P. & Dufrene, Y. F. Detection and localization of single molecular recognition events using atomic force microscopy. Nat. Methods3, 347–355 (2006). CASGoogle Scholar
- Ando, T., Uchihashi, T. & Kodera, N. High-speed AFM and applications to biomolecular systems. Ann. Rev. Biophys.42, 393–414 (2013). CASGoogle Scholar
- Dufrene, Y. F., Martinez-Martin, D., Medalsy, I., Alsteens, D. & Muller, D. J. Multiparametric imaging of biological systems by force-distance curve–based AFM. Nat. Methods10, 847–854 (2013). CASGoogle Scholar
- Garcia, R. & Proksch, R. Nanomechancial mapping of soft matter by bimodal force microscopy. Eur. Polym. J.49, 1897–1906 (2013). CASGoogle Scholar
- Radmacher, M., Tillmann, R. W., Fritz, M. & Gaub, H. E. From molecules to cells: imaging soft samples with the atomic force microscope. Science257, 1900–1905 (1992). CASGoogle Scholar
- Henderson, E., Haydon, P. G. & Sakaguchi, D. S. Actin filament dynamics in living glial cells imaged by atomic force microscopy. Science257, 1944–1946 (1992). CASGoogle Scholar
- Hoh, J. H. & Schoenenberger, C. A. Surface morphology and mechanical properties of MDCK monolayers by atomic force microscopy. J. Cell Sci.107, 1105–1114 (1994). Google Scholar
- Hoh, J. H., Lal, R., John, S. A., Revel, J.-P. & Arnsdorf, M. F. Atomic force microscopy and dissection of gap junctions. Science253, 1405–1408 (1991). CASGoogle Scholar
- Mou, J., Yang, J. & Shao, Z. Atomic force microscopy of cholera toxin B-oligomers bound to bilayers of biologically relevant lipids. J. Mol. Biol.248, 507–512 (1995). CASGoogle Scholar
- Schabert, F. A., Henn, C. & Engel, A. Native Escherichia coli OmpF porin surfaces probed by atomic force microscopy. Science268, 92–94 (1995). CASGoogle Scholar
- Hansma, H. G. et al. Reproducible imaging and dissection of plasmid DNA under liquid with the atomic force microscope. Science256, 1180–1184 (1992). CASGoogle Scholar
- Egger, M. et al. Wet lipid protein membranes imaged at submolecular resolution by atomic force microscopy. J. Struct. Biol.103, 89–94 (1990). CASGoogle Scholar
- Zasadzinski, J. A., Viswanathan, R., Madsen, L., Garnaes, J. & Schwartz, D. K. Langmuir-Blodgett films. Science263, 1726–1733 (1994). CASGoogle Scholar
- Yang, J., Mou, J. X. & Shao, Z. F. Structure and stability of pertussis toxin studied by in situ atomic force microscopy. FEBS Lett.338, 89–92 (1994). CASGoogle Scholar
- Müller, D. J., Schabert, F. A., Büldt, G. & Engel, A. Imaging purple membranes in aqueous solutions at sub-nanometer resolution by atomic force microscopy. Biophys. J.68, 1681–1686 (1995). Google Scholar
- Müller, D. J., Engel, A., Carrascosa, J. & Veléz, M. The bacteriophage phi29 head–tail connector imaged at high resolution with atomic force microscopy in buffer solution. EMBO J.16, 2547–2553 (1997). Google Scholar
- Czajkowsky, D. M., Sheng, S. & Shao, Z. Staphylococcal alpha-hemolysin can form hexamers in phospholipid bilayers. J. Mol. Biol.276, 325–330 (1998). CASGoogle Scholar
- Seelert, H. et al. Proton powered turbine of a plant motor. Nature405, 418–419 (2000). CASGoogle Scholar
- Scheuring, S., Reiss-Husson, F., Engel, A., Rigaud, J. L. & Ranck, J. L. High-resolution AFM topographs of Rubrivivax gelatinosus light- harvesting complex LH2. EMBO J.20, 3029–3035 (2001). CASGoogle Scholar
- Fotiadis, D. et al. Atomic-force microscopy: rhodopsin dimers in native disc membranes. Nature421, 127–128 (2003). CASGoogle Scholar
- Goldsbury, C., Kistler, J., Aebi, U., Arvinte, T. & Cooper, G. J. Watching amyloid fibrils grow by time-lapse atomic force microscopy. J. Mol. Biol.285, 33–39 (1999). CASGoogle Scholar
- Bezanilla, M. et al. Motion and enzymatic degradation of DNA in the atomic force microscope. Biophys. J.67, 2454–2459 (1994). CASGoogle Scholar
- Grandbois, M., Clausen-Schaumann, H. & Gaub, H. Atomic force microscope imaging of phospholipid bilayer degradation by phospholipase A2. Biophys. J.74, 2398–2404 (1998). CASGoogle Scholar
- Muller, D. J. & Engel, A. Voltage and pH-induced channel closure of porin OmpF visualized by atomic force microscopy. J. Mol. Biol.285, 1347–1351 (1999). CASGoogle Scholar
- Müller, D. J., Hand, G. M., Engel, A. & Sosinsky, G. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J.21, 3598–3607 (2002). This paper reports using AFM to image animal communication channels at work with high-resolution.Google Scholar
- Stoffler, D., Goldie, K. N., Feja, B & Aebi, U. Calcium-mediated structural changes of native nuclear pore complexes monitored by time-lapse atomic force microscopy. J. Mol. Biol.287, 741–752 (1999). CASGoogle Scholar
- Czajkowsky, D. M., Hotze, E. M., Shao, Z. & Tweten, R. K. Vertical collapse of a cytolysin prepore moves its transmembrane beta-hairpins to the membrane. EMBO J.23, 3206–3215 (2004). CASGoogle Scholar
- Scheuring, S. & Sturgis, J. N. Chromatic adaptation of photosynthetic membranes. Science309, 484–487 (2005). CASGoogle Scholar
- Engel, A. & Muller, D. J. Observing single biomolecules at work with the atomic force microscope. Nat. Struct. Biol.7, 715–718 (2000). CASGoogle Scholar
- Albrecht, T. R., Grutter, P., Horne, D. & Rugar, D. Frequency-modulation detection using high-Q cantilevers for enhanced force microscope sensitivity. J. Appl. Phys.69, 668–673 (1991). Google Scholar
- Putman, C. A. J., Vanderwerf, K. O., Degrooth, B. G., Vanhulst, N. F. & Greve, J. Tapping mode atomic-force microscopy in liquid. Appl. Phys. Lett.64, 2454–2456 (1994). CASGoogle Scholar
- Garcia, R. & Herruzo, E. T. The emergence of multifrequency force microscopy. Nat. Nanotech.7, 217–226 (2012). A review describing recent progress in multifrequency force microscopy, and discussing its potential for studying proteins and cells.CASGoogle Scholar
- Hansma, P. K. et al. Tapping mode atomic-force microscopy in liquids. Appl. Phys. Lett.64, 1738–1740 (1994). CASGoogle Scholar
- Wegmann, S. et al. Human Tau isoforms assemble into ribbon-like fibrils that display polymorphic structure and stability. J. Biol. Chem.285, 27302–27313 (2010). CASGoogle Scholar
- Ido, S. et al. Beyond the helix pitch: direct visualization of native DNA in aqueous solution. ACS Nano7, 1817–1822 (2013). CASGoogle Scholar
- Ido, S. et al. Immunoactive two-dimensional self-assembly of monoclonal antibodies in aqueous solution revealed by atomic force microscopy. Nat. Mater.13, 264–270 (2014). CASGoogle Scholar
- Möller, C., Allen, M., Elings, V., Engel, A. & Müller, D. J. Tapping mode atomic force microscopy produces faithful high-resolution images of protein surfaces. Biophys. J.77, 1050–1058 (1999). Google Scholar
- Stark, M., Moller, C., Muller, D. J. & Guckenberger, R. From images to interactions: high-resolution phase imaging in tapping-mode atomic force microscopy. Biophys. J.80, 3009–3018 (2001). CASGoogle Scholar
- Kasas, S. & Ikai, A. A method for anchoring round shaped cells for atomic force microscope imaging. Biophys. J.68, 1678–1680 (1995). CASGoogle Scholar
- Andre, G. et al. Imaging the nanoscale organization of peptidoglycan in living Lactococcus lactis cells. Nat. Commun.1, 27 (2010). Google Scholar
- Hansma, P. K., Drake, B., Marti, O., Gould, S. A. & Prater, C. B. The scanning ion-conductance microscope. Science243, 641–643 (1989). CASGoogle Scholar
- Novak, P. et al. Nanoscale live-cell imaging using hopping probe ion conductance microscopy. Nat. Methods6, 279–281 (2009). CASGoogle Scholar
- Drake, B., Randall, C., Bridges, D. & Hansma, P. K. A new ion sensing deep atomic force microscope. Rev. Sci. Instrum.85, 083706 (2014). Google Scholar
- Roos, W. H., Bruinsma, R. & Wuite, G. J. L. Physical virology. Nat. Phys.6, 733–743 (2010). CASGoogle Scholar
- Oesterhelt, F. et al. Unfolding pathways of individual bacteriorhodopsins. Science288, 143–146 (2000). CASGoogle Scholar
- Kufer, S. K., Puchner, E. M., Gumpp, H., Liedl, T. & Gaub, H. E. Single-molecule cut-and-paste surface assembly. Science319, 594–596 (2008). CASGoogle Scholar
- Braunschweig, A. B., Huo, F. & Mirkin, C. A. Molecular printing. Nat. Chem.1, 353–358 (2009). CASGoogle Scholar
- Cattin, C. J. et al. Mechanical control of mitotic progression in single animal cells. Proc. Natl Acad. Sci. USA112, 11258–11263 (2015). CASGoogle Scholar
- Butt, H. J., Cappella, B. & Kappl, M. Force measurements with the atomic force microscope: technique, interpretation and applications. Surf. Sci. Rep.59, 1–152 (2005). CASGoogle Scholar
- Dong, M., Husale, S. & Sahin, O. Determination of protein structural flexibility by microsecond force spectroscopy. Nat. Nanotech.4, 514–517 (2009). CASGoogle Scholar
- Martinez-Martin, D., Herruzo, E. T., Dietz, C., Gomez-Herrero, J. & Garcia, R. Noninvasive protein structural flexibility mapping by bimodal dynamic force microscopy. Phys. Rev. Lett.106, 198101 (2011). CASGoogle Scholar
- Herruzo, E. T., Perrino, A. P. & Garcia, R. Fast nanomechanical spectroscopy of soft matter. Nat. Commun.5, 3126 (2014). Google Scholar
- Preiner, J. et al. High-speed AFM images of thermal motion provide stiffness map of interfacial membrane protein moieties. Nano Lett.15, 759–763 (2015). CASGoogle Scholar
- Radmacher, M., Cleveland, J. P., Fritz, M., Hansma, H. G. & Hansma, P. K. Mapping interaction forces with the atomic force microscope. Biophys. J.66, 2159–2165 (1994). CASGoogle Scholar
- Heinz, W. F. & Hoh, J. H. Spatially resolved force spectroscopy of biological surfaces using the atomic force microscope. Trends Biotechnol.17, 143–150 (1999). CASGoogle Scholar
- Rotsch, C. & Radmacher, M. Drug-induced changes of cytoskeletal structure and mechanics in fibroblasts: an atomic force microscopy study. Biophys. J.78, 520–535 (2000). CASGoogle Scholar
- Matzke, R., Jacobson, K. & Radmacher, M. Direct, high-resolution measurement of furrow stiffening during division of adherent cells. Nat. Cell Biol.3, 607–610 (2001). CASGoogle Scholar
- Plodinec, M. et al. The nanomechanical signature of breast cancer. Nat. Nanotech.7, 757–765 (2012). CASGoogle Scholar
- Rebelo, L. M., de Sousa, J. S., Mendes Filho, J. & Radmacher, M. Comparison of the viscoelastic properties of cells from different kidney cancer phenotypes measured with atomic force microscopy. Nanotechnology24, 055102 (2013). CASGoogle Scholar
- Touhami, A., Nysten, B. & Dufrêne, Y. F. Nanoscale mapping of the elasticity of microbial cells by atomic force microscopy. Langmuir19, 4539–4543 (2003). CASGoogle Scholar
- Viani, M. B. et al. Fast imaging and fast force spectroscopy of single biopolymers with a new atomic force microscope designed for small cantilevers. Rev. Sci. Instrum.70, 4300–4303 (1999). This paper reports the invention of small cantilevers for fast AFM imaging and force spectroscopy.CASGoogle Scholar
- Viani, M. B. et al. Small cantilevers for force spectroscopy of single molecules. J. Appl. Phys.86, 2258–2262 (1999). CASGoogle Scholar
- Ando, T. et al. A high-speed atomic force microscope for studying biological macromolecules. Proc. Natl Acad. Sci. USA98, 12468–12472 (2001). CASGoogle Scholar
- Alcaraz, J. et al. Correction of microrheological measurements of soft samples with atomic force microscopy for the hydrodynamic drag on the cantilever. Langmuir18, 716–721 (2002). CASGoogle Scholar
- Sahin, O., Magonov, S., Su, C., Quate, C. F. & Solgaard, O. An atomic force microscope tip designed to measure time-varying nanomechanical forces. Nat. Nanotech.2, 507–514 (2007). Google Scholar
- Medalsy, I., Hensen, U. & Muller, D. J. Imaging and quantifying chemical and physical properties of native proteins at molecular resolution by force-volume AFM. Angew. Chem. Int. Ed.50, 12103–12108 (2011). CASGoogle Scholar
- Sullan, R. M., Li, J. K. & Zou, S. Direct correlation of structures and nanomechanical properties of multicomponent lipid bilayers. Langmuir25, 7471–7477 (2009). CASGoogle Scholar
- Heu, C., Berquand, A., Elie-Caille, C. & Nicod, L. Glyphosate-induced stiffening of HaCaT keratinocytes, a Peak Force Tapping study on living cells. J. Struct. Biol.178, 1–7 (2012). CASGoogle Scholar
- Formosa-Dague, C., Speziale, P., Foster, T. J., Geoghegan, J. A. & Dufrene, Y. F. Zinc-dependent mechanical properties of Staphylococcus aureus biofilm-forming surface protein SasG. Proc. Natl Acad. Sci. USA113, 410–415 (2016). CASGoogle Scholar
- Alsteens, D., Trabelsi, H., Soumillion, P. & Dufrene, Y. F. Multiparametric atomic force microscopy imaging of single bacteriophages extruding from living bacteria. Nat. Commun.4, 2926 (2013). Google Scholar
- Carrasco, C. et al. Built-in mechanical stress in viral shells. Biophys. J.100, 1100–1108 (2011). CASGoogle Scholar
- Zink, M. & Grubmuller, H. Mechanical properties of the icosahedral shell of southern bean mosaic virus: a molecular dynamics study. Biophys. J.96, 1350–1363 (2009). CASGoogle Scholar
- Carrasco, C. et al. DNA-mediated anisotropic mechanical reinforcement of a virus. Proc. Natl Acad. Sci. USA103, 13706–13711 (2006). CASGoogle Scholar
- Alsteens, D., Garcia, M. C., Lipke, P. N. & Dufrene, Y. F. Force-induced formation and propagation of adhesion nanodomains in living fungal cells. Proc. Natl Acad. Sci. USA107, 20744–20749 (2010). This paper reports using recognition imaging to demonstrate that microbial cell adhesion proteins form nanoclusters under mechanical force.CASGoogle Scholar
- Pfreundschuh, M., Hensen, U. & Muller, D. J. Quantitative imaging of the electrostatic field and potential generated by a transmembrane protein pore at subnanometer resolution. Nano Lett.13, 5585–5593 (2013). CASGoogle Scholar
- Evans, E. A. & Calderwood, D. A. Forces and bond dynamics in cell adhesion. Science316, 1148–1153 (2007). CASGoogle Scholar
- Medalsy, I. D. & Muller, D. J. Nanomechanical properties of proteins and membranes depend on loading rate and electrostatic interactions. ACS Nano7, 2642–2650 (2013). CASGoogle Scholar
- Frisbie, C. D., Rozsnyai, L. F., Noy, A., Wrighton, M. S. & Lieber, C. M. Functional group imaging by chemical force microscopy. Science265, 2071–2074 (1994). CASGoogle Scholar
- Hinterdorfer, P., Baumgartner, W., Gruber, H. J., Schilcher, K. & Schindler, H. Detection and localization of individual antibody-antigen recognition events by atomic force microscopy. Proc. Natl Acad. Sci. USA93, 3477–3481 (1996). CASGoogle Scholar
- Ludwig, M., Dettmann, W. & Gaub, H. E. Atomic force microscope imaging contrast based on molecular recognition. Biophys. J.72, 445–448 (1997). CASGoogle Scholar
- Grandbois, M., Dettmann, W., Benoit, M. & Gaub, H. E. Affinity imaging of red blood cells using an atomic force microscope. J. Histochem. Cytochem.48, 719–724 (2000). CASGoogle Scholar
- Florin, E. L., Moy, V. T. & Gaub, H. E. Adhesion forces between individual ligand-receptor pairs. Science264, 415–417 (1994). CASGoogle Scholar
- Lee, G. U., Chrisey, L. A. & Colton, R. J. Direct measurement of the forces between complementary strands of DNA. Science266, 771–773 (1994). CASGoogle Scholar
- Kienberger, F. et al. Recognition force spectroscopy studies of the NTA-His6 bond. Single Mol.1, 59–65 (2000). CASGoogle Scholar
- Thie, M. et al. Interactions between trophoblast and uterine epithelium: monitoring of adhesive forces. Hum. Reprod.13, 3211–3219 (1998). CASGoogle Scholar
- Kim, H., Arakawa, H., Osada, T. & Ikai, A. Quantification of cell adhesion force with AFM: distribution of vitronectin receptors on a living MC3T3-E1 cell. Ultramicroscopy97, 359–363 (2003). CASGoogle Scholar
- Kim, H. et al. Quantification of the number of EP3 receptors on a living CHO cell surface by the AFM. Ultramicroscopy106, 652–662 (2006). CASGoogle Scholar
- Roduit, C. et al. Elastic membrane heterogeneity of living cells revealed by stiff nanoscale membrane domains. Biophys. J.94, 1521–1532 (2008). CASGoogle Scholar
- Alsteens, D. et al. Imaging G protein–coupled receptors while quantifying their ligand-binding free-energy landscape. Nat. Methods12, 845–851 (2015). This paper showed that attaching a ligand to the AFM stylus allows it to image and map its binding to human G protein–coupled receptors and to reconstruct the ligand-binding free-energy landscape.CASGoogle Scholar
- Andre, G. et al. Fluorescence and atomic force microscopy imaging of wall teichoic acids in Lactobacillus plantarum. ACS Chem. Biol.6, 366–376 (2011). CASGoogle Scholar
- Dupres, V. et al. Nanoscale mapping and functional analysis of individual adhesins on living bacteria. Nat. Methods2, 515–520 (2005). This paper reports that AFM tips labelled with bioligands can map the distribution of single adhesion proteins on bacterial pathogens and reveal their assembly into nanodomains.CASGoogle Scholar
- Pfreundschuh, M. et al. Identifying and quantifying two ligand-binding sites while imaging native human membrane receptors by AFM. Nat. Commun.6, 8857 (2015). CASGoogle Scholar
- Raab, A. et al. Antibody recognition imaging by force microscopy. Nat. Biotechnol.17, 901–905 (1999). CASGoogle Scholar
- Stroh, C. et al. Single-molecule recognition imaging microscopy. Proc. Natl Acad. Sci. USA101, 12503–12507 (2004). CASGoogle Scholar
- Chtcheglova, L. A., Waschke, J., Wildling, L., Drenckhahn, D. & Hinterdorfer, P. Nano-scale dynamic recognition imaging on vascular endothelial cells. Biophys. J.93, L11–L13 (2007). CASGoogle Scholar
- Zhang, S., Aslan, H., Besenbacher, F. & Dong. M. Quantitative biomolecular imaging by dynamic nanomechanical mapping. Chem. Soc. Rev.43, 7412–7429 (2014). CASGoogle Scholar
- Dietz, C., Herruzo, E. T., Lozano, J. R. & Garcia, R. Nanomechanical coupling enables detection and imaging of 5 nm superparamagnetic particles in liquid. Nanotechnology22, 125708 (2011). Google Scholar
- Herruzo, E. T., Asakawa, H., Fukuma, T. & Garcia, R. Three-dimensional quantitative force maps in liquid with 10 piconewton, angstrom and sub-minute resolutions. Nanoscale5, 2678–2685 (2013). CASGoogle Scholar
- Fukuma, T., Higgins, M. J. & Jarvis, S. P. Direct imaging of individual intrinsic hydration layers on lipid bilayers at Angstrom resolution. Biophys. J.92, 3603–3609 (2007). CASGoogle Scholar
- Cartagena, A., Hernando-Perez, M., Carrascosa, J. L., de Pablo, P. J. & Raman, A. Mapping in vitro local material properties of intact and disrupted virions at high resolution using multi-harmonic atomic force microscopy. Nanoscale5, 4729–4736 (2013). CASGoogle Scholar
- Cartagena-Rivera, A. X., Wang, W. H., Geahlen, R. L. & Raman, A. Fast, multi-frequency, and quantitative nanomechanical mapping of live cells using the atomic force microscope. Sci. Rep.5, 11692 (2015). CASGoogle Scholar
- Kim, D. & Sahin, O. Imaging and three-dimensional reconstruction of chemical groups inside a protein complex using atomic force microscopy. Nat. Nanotech.10, 264–269 (2015). CASGoogle Scholar
- Shekhawat, G. S. & Dravid, V. P. Nanoscale imaging of buried structures via scanning near-field ultrasound holography. Science310, 89–92 (2005). CASGoogle Scholar
- Tetard, L. et al. Imaging nanoparticles in cells by nanomechanical holography. Nat. Nanotech.3, 501–505 (2008). CASGoogle Scholar
- Verbiest, G. J. & Rost, M. J. Beating beats mixing in heterodyne detection schemes. Nat. Commun.6, 6444 (2015). CASGoogle Scholar
- Kindt, J. H., Fantner, G. E., Cutroni, J. A. & Hansma, P. K. Rigid design of fast scanning probe microscopes using finite element analysis. Ultramicroscopy100, 259–265 (2004). CASGoogle Scholar
- Ando, T., Uchihashi, T. & Fukuma, T. High-speed atomic force microscopy for nano-visualization of dynamic biomolecular processes. Prog. Surf. Sci.83, 337–437 (2008). CASGoogle Scholar
- Kodera, M., Yamashita, H. & Ando, T. Active damping of the scanner for high-speed atomic force microscopy. Rev. Sci. Instrum.76, 053708 (2005). Google Scholar
- Kodera, N., Sakashita, M. & Ando, T. Dynamic proportional-integral-differential controller for high-speed atomic force microscopy. Rev. Sci. Instrum.77, 083704 (2006). Google Scholar
- Viani, M. B. et al. Probing protein–protein interactions in real time. Nat. Struct. Biol.7, 644–647 (2000). CASGoogle Scholar
- Ando, T. et al. A high-speed atomic force microscope for studying biological macromolecules in action. ChemPhysChem4, 1196–1202 (2003). CASGoogle Scholar
- Shibata, M., Yamashita, H., Uchihashi, T., Kandori, H. & Ando, T. High-speed atomic force microscopy shows dynamic molecular processes in photoactivated bacteriorhodopsin. Nat. Nanotech.5, 208–212 (2010). CASGoogle Scholar
- Kodera, N., Yamamoto, D., Ishikawa, R. & Ando, T. Video imaging of walking myosin V by high-speed atomic force microscopy. Nature468, 72–76 (2010). This paper showed that high-speed AFM can be used to watch proteins functioning in real-time.CASGoogle Scholar
- Uchihashi, T., Iino, R., Ando, T. & Noji, H. High-speed atomic force microscopy reveals rotary catalysis of rotorless F(1)-ATPase. Science333, 755–758 (2011). CASGoogle Scholar
- Chiaruttini, N. et al. Relaxation of loaded ESCRT-III spiral springs drives membrane deformation. Cell163, 866–879 (2015). CASGoogle Scholar
- Sakiyama, Y., Mazur, A., Kapinos, L. E. & Lim, R. Y. Spatiotemporal dynamics of the nuclear pore complex transport barrier resolved by high-speed atomic force microscopy. Nat. Nanotech.11, 719–723 (2016). CASGoogle Scholar
- Fantner, G. E., Barbero, R. J., Gray, D. S. & Belcher, A. M. Kinetics of antimicrobial peptide activity measured on individual bacterial cells using high-speed atomic force microscopy. Nat. Nanotech.5, 280–285 (2010). CASGoogle Scholar
- Yamashita, H. et al. Single-molecule imaging on living bacterial cell surface by high-speed AFM. J. Mol. Biol.422, 300–309 (2012). CASGoogle Scholar
- Shibata, M., Uchihashi, T., Ando, T. & Yasuda, R. Long-tip high-speed atomic force microscopy for nanometer-scale imaging in live cells. Sci. Rep.5, 8724 (2015). CASGoogle Scholar
- Uchihashi, T., Watanabe, H., Fukuda, S., Shibata, M. & Ando, T. Functional extension of high-speed AFM for wider biological applications. Ultramicroscopy160, 182–196 (2016). CASGoogle Scholar
- El-Kirat-Chatel, S. & Dufrene, Y. F. Nanoscale imaging of the Candida — macrophage interaction using correlated fluorescence-atomic force microscopy. ACS Nano6, 10792–10799 (2012). CASGoogle Scholar
- Sharma, A., Anderson, K. & Muller, D. J. Actin microridges characterized by laser scanning confocal and atomic force microscopy. FEBS Lett.579, 2001–2009 (2005). CASGoogle Scholar
- Schillers, H., Medalsy, I., Hu, S., Slade, A. L. & Shaw, J. E. PeakForce Tapping resolves individual microvilli on living cells. J. Mol. Recognit.29, 95–101 (2016). CASGoogle Scholar
- Benoit, M., Gabriel, D., Gerisch, G. & Gaub, H. E. Discrete interactions in cell adhesion measured by single-molecule force spectroscopy. Nat. Cell Biol.2, 313–317 (2000). CASGoogle Scholar
- Krieg, M. et al. Tensile forces govern germ-layer organization in zebrafish. Nat. Cell Biol.10, 429–436 (2008). CASGoogle Scholar
- Cuerrier, C. M., Gagner, A., Lebel, R., Gobeil, F. Jr & Grandbois, M. Effect of thrombin and bradykinin on endothelial cell mechanical properties monitored through membrane deformation. J. Mol. Recognit.22, 389–396 (2009). CASGoogle Scholar
- Pelling, A. E., Veraitch, F. S., Chu, C. P., Mason, C. & Horton, M. A. Mechanical dynamics of single cells during early apoptosis. Cell Motil. Cytoskel.66, 409–422 (2009). CASGoogle Scholar
- Ramanathan, S. P. et al. Cdk1-dependent mitotic enrichment of cortical myosin II promotes cell rounding against confinement. Nat. Cell Biol.17, 148–159 (2015). CASGoogle Scholar
- Stewart, M. P. et al. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature469, 226–230 (2011). CASGoogle Scholar
- Duman, M. et al. Improved localization of cellular membrane receptors using combined fluorescence microscopy and simultaneous topography and recognition imaging. Nanotechnology21, 115504 (2010). CASGoogle Scholar
- Lipke, P. N. et al. Strengthening relationships: amyloids create adhesion nanodomains in yeasts. Trends Microbiol.20, 59–65 (2012). CASGoogle Scholar
- Alsteens, D. et al. Nanomechanical mapping of first binding steps of a virus to animal cells. Nat. Nanotech.12, 177–183 (2017). This paper showed that attaching a rabies virus to the AFM stylus allows living animal cells to be imaged with confocal microscopy and AFM, to simultaneously localize virus-binding, and to quantify the virus-binding process and free-energy landscape.CASGoogle Scholar
- Churnside, A. B. & Perkins, T. T. Ultrastable atomic force microscopy: improved force and positional stability. FEBS Lett.588, 3621–3630 (2014). CASGoogle Scholar
- King, G. M., Carter, A. R., Churnside, A. B., Eberle, L. S. & Perkins, T. T. Ultrastable atomic force microscopy: atomic-scale stability and registration in ambient conditions. Nano Lett.9, 1451–1456 (2009). CASGoogle Scholar
- Franz, C. M. & Muller, D. J. Analysing focal adhesion structure by AFM. J. Cell Sci.118, 5315–5323 (2005). CASGoogle Scholar
- Lucas, R. W., Kuznetsov, Y. G., Larson, S. B. & McPherson, A. Crystallization of Brome mosaic virus and T = 1 Brome mosaic virus particles following a structural transition. Virology286, 290–303 (2001). CASGoogle Scholar
- Friedrichs, J., Taubenberger, A., Franz, C. M. & Muller, D. J. Cellular remodelling of individual collagen fibrils visualized by time-lapse AFM. J. Mol. Biol.372, 594–607 (2007). CASGoogle Scholar
- Mari, S. A. et al. Gating of the MlotiK1 potassium channel involves large rearrangements of the cyclic nucleotide-binding domains. Proc. Natl Acad. Sci. USA108, 20802–20807 (2011). Google Scholar
- Bestembayeva, A. et al. Nanoscale stiffness topography reveals structure and mechanics of the transport barrier in intact nuclear pore complexes. Nat. Nanotech.10, 60–64 (2015). CASGoogle Scholar
Acknowledgements
Y.F.D. was supported by the Université catholique de Louvain, the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (grant agreement no. 693630), the WELBIO (grant no. WELBIO-CR-2015A-05), the National Fund for Scientific Research (FNRS), the Federal Office for Scientific, Technical and Cultural Affairs (Interuniversity Poles of Attraction Programme) and the Research Department of the Communauté française de Belgique (Concerted Research Action). D.A. and D.M.M. were supported by the European Molecular Biology Organization (EMBO; ALTF 265-2013 and ALTF 506-2012). D.J.M. was supported by the Swiss National Science Foundation (SNF; grants 205320_160199 and 310030B_160225), the NCCR Molecular Systems Engineering and the Swiss Commission for Technology and Innovation (CTI; grant 17970.1). C.G. was supported by the Swiss Nano Institute (SNI) of the University of Basel. R.G. acknowledges financial support from the European Research Council AdG no. 340177 and the Ministerio de Economia y Competitividad MAT2016-76507-R. T.A. was supported by the Japan Society for the Promotion of Science (JSPS; grants 24227005 and 26119003) and by the Japan Science and Technology Agency (JST; CREST program on Structural Life Science and Advanced Core Technology for Innovative Life Science Research).
Author information
Authors and Affiliations
- Institute of Life Sciences and Walloon Excellence in Life Sciences and Biotechnology (WELBIO), Université catholique de Louvain, Croix du Sud 4-5, bte L7.07.06., Louvain-la-Neuve, B-1348, Belgium Yves F. Dufrêne & David Alsteens
- Department of Physics, Kanazawa University, Kanazawa, 920-1192, Japan Toshio Ando
- Instituto de Ciencia de Materiales de Madrid, CSIC, Sor Juana Inés de la Cruz 3, Madrid, 28049, Spain Ricardo Garcia
- Department of Biosystems Science and Engineering, Eidgenössische Technische Hochschule (ETH) Zürich, Mattenstrasse 28, Basel, 4056, Switzerland David Martinez-Martin & Daniel J. Müller
- Department of BioNanoscience, Delft University of Technology, Van der Maasweg 9, Delft, 2629 HZ, The Netherlands Andreas Engel
- Swiss Nanoscience Institute, University of Basel, Klingelbergstrasse 80, Basel, 4057, Switzerland Christoph Gerber
- Yves F. Dufrêne










